Explain This Difference in Boiling Points Chegg
Intermolecular force has a higher boiling point Look for functional groups that may indicate polar molecule. In boiling the motion of particles is increased and this force separates the particles apart from each other.
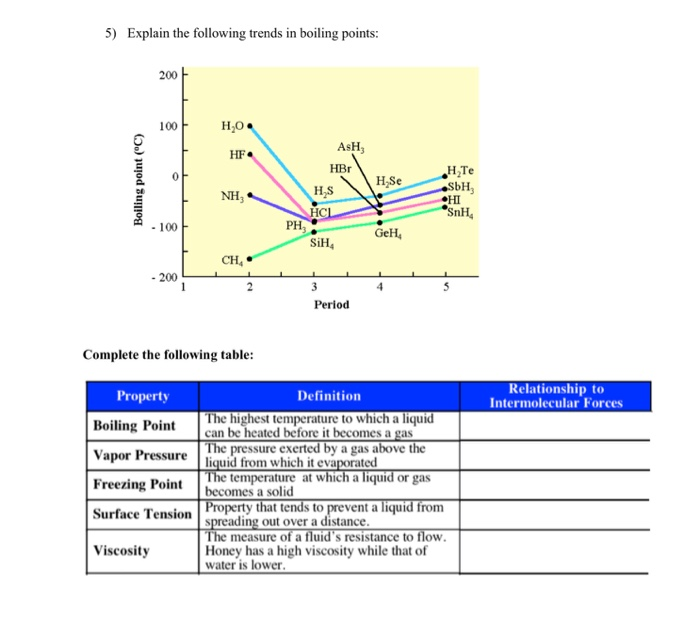
Solved 5 Explain The Following Trends In Boiling Points Chegg Com
This is because they are made up of molecules that are held together by the weak force of attraction thus less heat is required to break the force of attraction between these molecules and so they have low melting and boiling points.
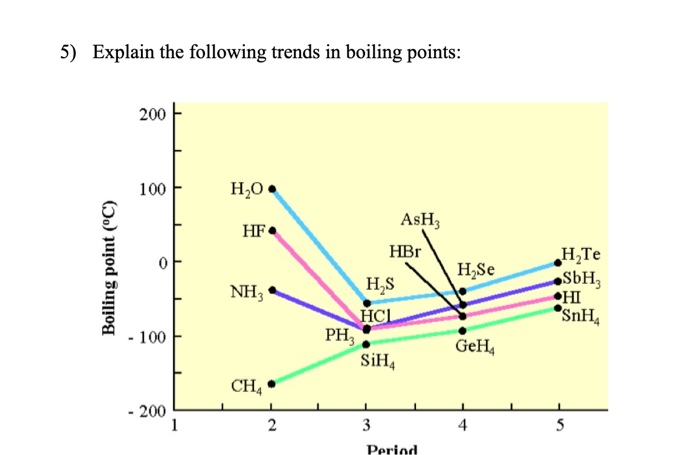
. The boiling point of water H2O is about 200C higher than the one would predict from the boiling points of H2S and H2Se. A HF 20C and HC -85C b Bra 59C and ICI 97C 3. The above extract from my book mentions clearly that branching makes the molecule more compact and thereby decreases the surface area.
Explain this difference in boiling points in terms of intermolecular forces. Ad Get 247 access to step-by-step textbook solutions and expert QA. The microscopic difference between evaporation and boiling is as follows.
Explain the difference in boiling points between the members of the following pairs of substances. Bp 85 degree C. Comparing the melting points of benzene and toluene you can see that the extra methyl group on toluene disrupts the molecules ability to stack thus decreasing the cumulative strength of intermolecular London dispersion forces.
Note also that the boiling point for toluene is 111 o C well above the boiling point of benzene 80 o C. Check Your Learning Ethane CH 3 CH 3 has a melting point of 183 C and a boiling point of 89 C. Explain this difference in boiling points in terms of heats of vaporization.
Determination of a Melting Point. C Water is less polar than H2S and H2Se. Which of the following statements best explains this apparent anomaly.
Therefore these molecules will be liquids at 25 25. Chemistry questions and answers. The temperature is uniform and the boiling also occurs throughout.
When a mixture of liquids is heated the components which have different boiling points go into gaseous phase at different instances. Predict the melting and boiling points for methylamine CH 3 NH 2. The boiling point of propane is 421 C the boiling point of dimethylether is 248 C and the boiling point of ethanol is 785 C.
The key difference between fractional and simple distillation is that fractional distillation is used w hen the components in the mixture have closer boiling points while simple distillation is used when the components in the mixture have a large difference in their boiling points. Chemical X has weaker intermolecular forces than Chemical Y 24. Distillation is a separation technique that is used to purify a liquid or a mixture of liquids by heating followed by cooling.
Bp 133 degree C. Refer to the graph on the right to answer the following questions. Use data from Table 125 to estimate a the boiling point of water in Santa Fe New Mexico if the prevailing atmospheric pressure is 640 mathrmmmHg b the prevailing atmospheric pressure at Lake Arrowhead California if the observed boiling.
Main Difference Fractional Distillation vs Simple Distillation. The key factor for the boiling point trend in. A Water has the lowest molecular weight b The H-O covalent bond is much stronger that the H-S and H-Se bonds.
Melting Point of Tetradecanol. Distillation is a physical separation method that is used to separate compounds from a. A Which liquid has the highest normal.
Explain the difference in their boiling points. A 211 mixture of 2-chloro-2-methylpropane MW. Who are the experts.
Between two nonpolar molecules of similar mass the more extended molecule will have the higher boiling point more extended à more surface area for London dispersion interaction. Explain the difference in boiling point between the isomers 1-chlorobutane and 2-methyi- 2-chloropropane in terms of structure. A HF 20C and HCI -85C b CHCI 61C and CHBr 150C c Br 59C and ICI 97C.
They are mostly non-conductor of electricity. Chemical X has a boiling point of 75 oC Chemical Y has a boiling point of 126 oC 23. From this experiment I learned that different substances have different boiling points and that the boilingmelting point of a substance can tell you about its identity.
Explain the difference in boiling points between the members of the following pairs of substances. The melting and boiling points of covalent compounds are usually low. 1-butanol has the higher boiling point because the molecules can form hydrogen bonds with each other It contains an OH bond.
The melting points of butanoic acid bromoethane and dimethyl ether are below 25 25 however the boiling points of all three molecules are above 25 25. Diethyl ether molecules do contain both oxygen atoms and hydrogen atoms. Melting Point of Tetradecanol C Trial 1.
CH 3CH 2CH 3 CH 3CH 2OH bp 42 C bp 78 C 2. In evaporation the movement of the particles is not the same. Condensation of the vapour allows it to be.
Consequently the boiling points of the branched chain alkanes are less than the straight chain isomers. Even for isomeric alkyl halides the boiling points decrease with branching.
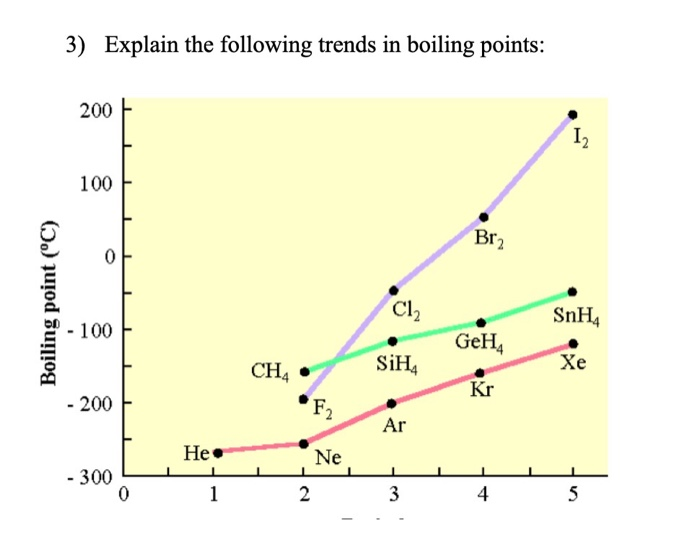
Solved 3 Explain The Following Trends In Boiling Points Chegg Com
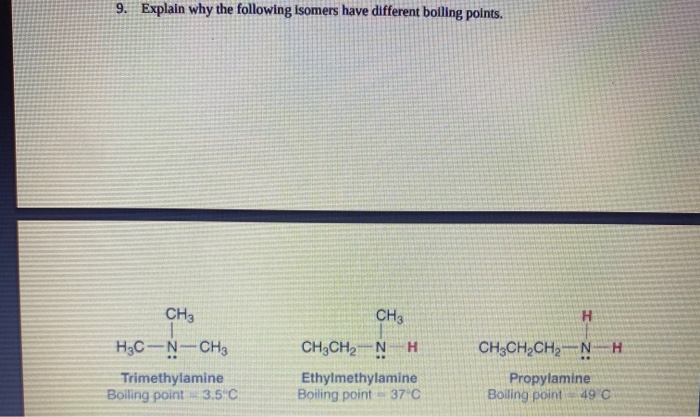
Solved 9 Explain Why The Following Isomers Have Different Chegg Com

Solved 3 Explain The Following Trends In Boiling Points Chegg Com
Comments
Post a Comment